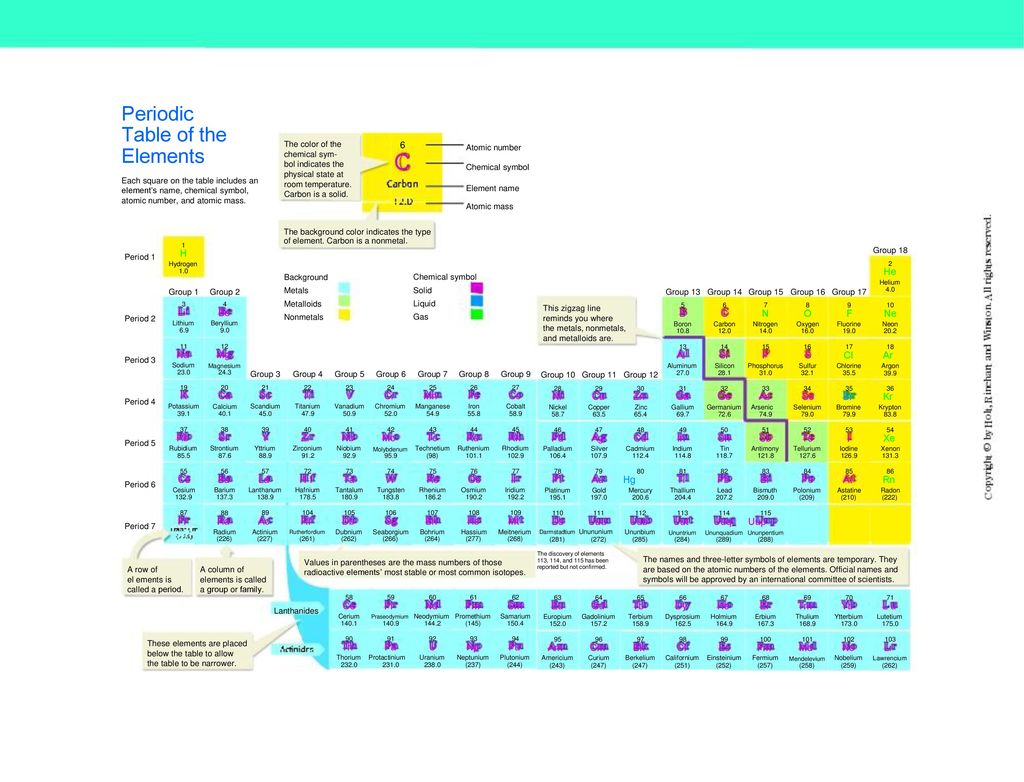
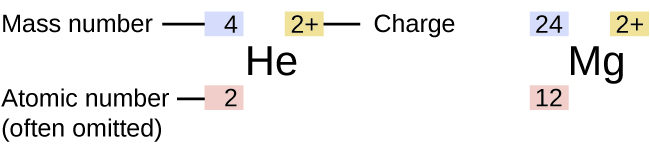In the periodic table above black squares indicate elements which are solids at room temperature about 22ºc those in blue squares are liquids at room temperature and those in red squares are gases at room temperature.
Magnesium element state at room temperature.
Other liquid elements.
Relative atomic mass.
An example is the halogen element chlorine.
Density is the mass of a substance that would fill 1 cm 3 at room temperature.
All group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
Classified as an alkaline earth metal magnesium is a solid at room temperature.
It can readily react with hot water and with oxygen at room temperature.
Properties sources and uses of the element magnesium.
It is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column group 2 or alkaline earth metals of the periodic table.
The sum of the oxidation states within a compound or ion must equal the overall charge.
Magnesium makes alloys with various metals which are strong and very light.
Magnesium is a chemical element with the symbol mg and atomic number 12.
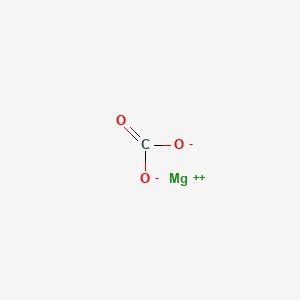
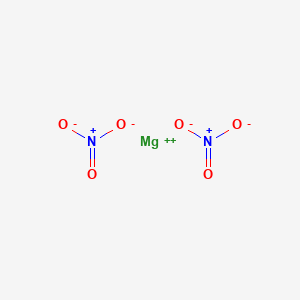
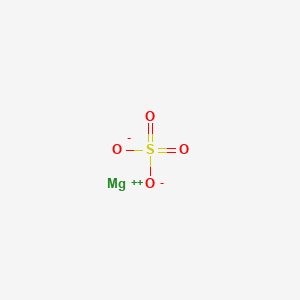
Some compounds include magnesium citrate magnesium oxide and magnesium chloride.
Nonetheless the use of mg alloys has been limited by their poor processability at room temperature and low corrosion resistance 2 2.
When pressure is controlled other pure elements may be found at room temperature.
Most of the metals are solids under ordinary conditions i e 25ºc 1 atmosphere of pressure etc with the exception of mercury hg element 80 which solidifies.
Phase at room temperature.
The melting temperature of magnesium is 651 c and its boiling point is 1100 c.
Although it is the eighth most abundant element in the universe and the seventh most abundant element in the earth s crust magnesium is never found free in nature magnesium was first isolated by sir humphry davy an english chemist through the electrolysis of a mixture of magnesium oxide mgo and mercuric oxide hgo in 1808.
Uncombined elements have an oxidation state of 0.
The first person to propose that magnesium was an element was joseph black of edinburgh in 1755 and.
Magnesium is a chemical element with symbol mg and atomic number 12.
That state of matter of an element may be predicted based on its phase diagram.
The compounds of magnesium are quite stable.